Have you ever tried to anneal some "mystery" steel or some air hardening steel by heating it to "critical" and
sticking it in your ash bucket or in some lime to slow its cooling rate enough to "soften" it? Have you noticed that for
certain steels it just doesn't work? Me, too.
Air hardening steels, in particular have to cool at pretty slow rates in order to anneal properly.
S7 is one example of such an air hardening steel alloy. I think it needs to cool from its "critical" of about 1850
degrees F. to about 1000 degrees F at a rate of 25 degrees per
hour or less. Some steels may need a cooling rate as slow as 10 degrees per hour to become fully annealed!
So, I wondered how well MY ash bucket works for this purpose. My ash bucket is a pail of about 3 gallons in size.
It is almost always overflowing because the butler doesn't empty it very often.
To test my own ash bucket as an annealling "oven", I slowly heated a 5 pound piece of truck axle to 1650 degrees F.
in my coal forge. Then I placed the part into the very center of the ashes with a thermocouple along side it,
not quite touching the part. I recorded the temperature about every 5 minutes.
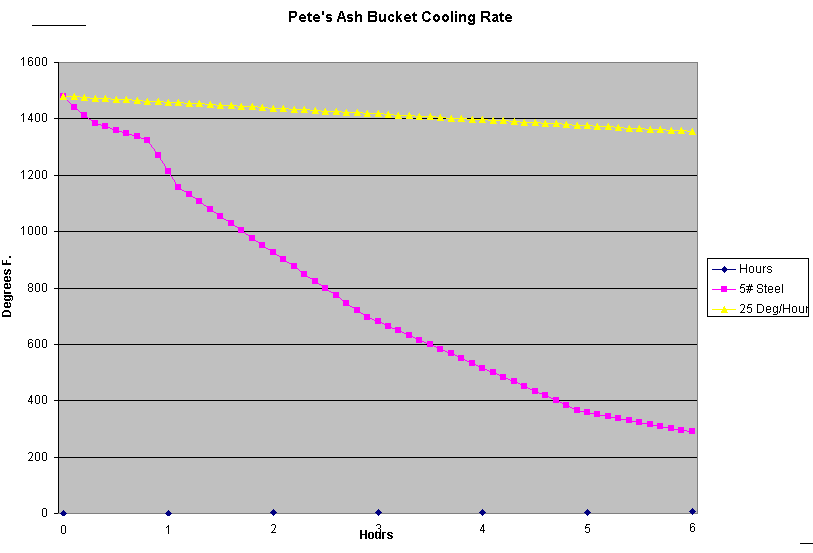
Notice that the temperature dropped about 300 degrees F in the first hour!
This is nowhere close to the required 25 degrees per hour that is necessary for many air hardening steels.
Plenty good for carbon steels. Works okay for 4140.
So, if you have wondered why you can't anneal that part that you spent 10 hours making, that's why.
I haven't tried this myself, but I have heard that the "old timers" used to build a good hot fire in the wood
stove, insert the parts to be annealed, add more wood, and let the whole thing burn out.
I'd assume that they got the parts up to temperature before they were put into the stove.
It sounds reasonable to me that if the fire took 6 or 8 hours to burn down, you might make it.
But I would want to test that cooling rate before committing an important piece to the stove.
A couple of other ways to achieve slow cooling:
-Put a heavy piece of steel in with small part.
-Preheat the ash bucket, particularly if the part is real small.
-Heat the part up in the forge, turn off the blower, and cover the whole thing with kaowool till it goes out.
Oh! So you noticed that the cooling rate on the graph changed abruptly at about 1350 degrees, did you?
I have been told that, as the structure of the steel changes upon cool-down(transformation),
an exothermic reaction occurs at that point, and this accounts for the REDUCTION in overall cooling rate for
a few minutes.
You can see this happen by watching a thin piece of high carbon steel (knife blade?) cooling in a very dark
place.
The hot steel looses all color, then, as the time goes forward, starts to glow a dull red before
loosing all color for good.
I hope this little experiment helps to answer a question or two for you.
I have recently tried using vermiculite instead of ashes because I thought the vermiculite would hold the heat better.
But my first test did not work out that way. I plan to run some more tests, first to verify that I did the first vermiculite
test correctly, then to try it with a coarser vermiculite than I now have.
Once I have run those tests, I will clean up this page a post a graph directly to it, along with my final conclusions.
In the meantime, I offer this excel spreadsheet, which has my initial findings:
Vermiculite Annealing Rate Spreadsheet
It is a messy spreadsheet, so don't work too hard to decipher some of the tables on the right hand side. The data on the
left and the graph should be all you need.